Precision oncology — a new era of tumor treatment
Bosch Research Blog | Posted by Christian Grumaz, 2024-06-26

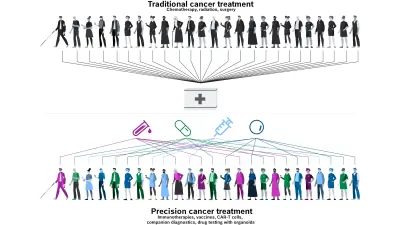
From “one size fits all” to “know your enemy” — the way tumors are diagnosed and treated is changing. Whereas cancer patients previously received a standard treatment, many tumors these days are genetically profiled and treated in a customized way. Dr. Christian Grumaz is a Senior Expert in the Life Science Group at Bosch Research, specializing in molecular diagnostics for infectious diseases and cancer. In this blog post, he answers the most important questions about precision oncology and offers insight into his work.
What are the advantages of precision medicine?
Every person — and every tumor — is unique. That is why treatment should be tailored as precisely as possible to the individual genetic and molecular characteristics of the patient. The vision of precision medicine is to provide the right treatment for the right patient at the right time. By analyzing inheritable genetic variations and specific tumors using cutting-edge methods, we can divide patients into narrowly defined groups and give them the appropriate treatment. This procedure — known as stratification — can lead to more effective treatment, fewer side-effects, and fewer unnecessary costs, while also improving healthcare provision overall. Alongside inheritable genetic variations that have an impact on the metabolization of medications, genetic markers in the tumor itself that determine treatment response or resistance are of particular interest.
What stage is precision oncology at today?
As things stand today, precision oncology is based on five pillars.
These tests serve to identify genetic changes in the tumor. This enables physicians to assess the type and prognosis of the tumor, identify resistances or companion diagnostics (CDx) that are significant for the effective use of the associated medication, and thus select a targeted treatment.
The selected medications specifically target the identified genetic changes and directly attack the resulting mutated proteins from the tumor. This is what is called targeted therapies, where “small drugs” or highly specific antibodies are used.
Certain treatments stimulate the patient’s immune system and are designed to directly attack tumor cells. One example is a type of drug that blocks proteins called checkpoint inhibitors that are expressed in some types of immune cells, such as T cells. These checkpoint inhibitors usually help to keep immune responses from being too strong and e.g. can keep T cells from killing cancer cells. However, when these checkpoints are blocked, T cells can kill cancer cells better.
This non-invasive method enables a closer monitoring of the treatment by analyzing the tumor DNA (ctDNA) and/or tumor cells (CTCs) circulating in the blood. Monitoring changes in the CTC enumeration and the ctDNA analysis enables physicians to monitor the progress of the illness and respond to changes in the tumor load.
By identifying genetic profiles, it is possible to prevent treatments of patients that are not effective for them, give rise to unnecessary costs, and potentially also involve life-threatening side-effects for them.
How do you genetically analyze a tumor?
If cancer is suspected, a tissue sample (the biopsy material) is examined for genetic characteristics in addition to the conventional histological tests. The DNA — and sometimes also the RNA — of the tumor is isolated and sections of the genome that are of particular interest are sequenced. These relevant sections can vary depending on the type of tumor. This is known as a gene panel — a group of genes that include genetic biomarkers. Basically, the greater the number of treatment options that are known to correlate with the identified biomarkers, the larger the gene panel is. It can range from a few small sections to several hundred. This kind of panel approach is now in widespread use and is superior to individual gene analyses in both technical and economic terms. Gene panels are currently the workhorses in the field of precision oncology.
What information do the gene panels provide?
A typical gene panel is generally made up of tumor-specific and tumor-agnostic markers that normally provide information about the stage of the cancer, the prognosis, and the best possible treatment options. On the one hand, highly specific biomarkers are required for better tumor classification and prognosis; on the other hand, the gene panels also include common biomarkers that identify mutations across different tumor types. In the latter case, tumor-agnostic can be used for these shared biomarkers.
In this way, customized, molecular tumor profiles can be compiled for each individual patient, and this profile plays a vital role in clinical decision-making for cancer treatment. These are known as “molecularly guided therapy options” (MGTO). They are generally discussed in expert groups (known as “tumor boards”), which focus on the patient and their specific findings.
What advances are being made in precision medicine?
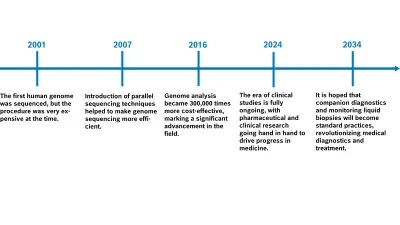
Since the advent of high-throughput sequencing technologies in around 2007, it has been possible to sequence genetic material cost-effectively — this is known as next-generation sequencing (NGS). This technology first of all promoted the identification of relevant biomarkers to support medicine development. NGS is now also becoming an increasingly important part of clinical routines when it comes to initial tumor characterization from tissue biopsy material (primary diagnosis), and above all also in treatment monitoring via liquid biopsies. The number of biomarkers and biomarker classes has been rising constantly ever since, as has the number of targeted therapy options. After all, many clinical studies involve the parallel development of new treatments with the correlating CDx biomarkers. We’re talking about a new era of clinical studies — next-generation sequencing (NGS) has triggered a biotechnological and information technology revolution.
There are already established standard gene panels for some types of tumor. One example is a type of drug that blocks proteins called checkpoints that are expressed in some types of immune cells, such as T cells. These checkpoints usually help to keep immune responses from being too strong and e.g. can keep T cells from killing cancer cells. However, when these checkpoint inhibitors are blocked, T cells can kill cancer cells better.
Even if not all patients are benefiting from this panel sequencing yet, it still helps to initiate targeted treatment in more than 70 percent of cases. The overall survival rate is rising significantly, from 11.4 months with conventional treatment to 18.6 months (Singal et al. 2019). The more clinical studies are conducted for medication development and the associated biomarker identification, the greater the scientific advances are. The panel is constantly expanding, so more and more patients are benefiting from the analyses.
What are the limits on precision medicine currently?
There are still a range of factors that make this form of oncology more difficult.
Access to the diagnostics is so far unevenly distributed. For example, 23 percent of NSCLC patients do not receive any CDx diagnostics, although the guidelines specify that they should (Mateo et. al. 2022). The patient-centered approach is often lacking.
The standard of care in precision oncology is constantly being updated on the basis of the latest scientific research in order to achieve the best outcomes for cancer patients. The challenge in this is ensuring that clinical awareness, policy making, and infrastructure can keep pace with the remarkable scientific advances so medical innovations are immediately put into practice in our healthcare systems.
Reimbursement and approvals vary greatly from country to country and region to region.
Analyses are complex and can only be conducted with high-tech equipment and well-trained personnel. In general, this can only be done by larger cancer centers on-site. Or for external examination, samples are sent away to central laboratories or to laboratories that are specialized in certain tumor types.
Only a few hospitals have a tumor board with experts that feel confident enough to deal with the complexity of genome analyses.
Turnaround times and costs are still comparatively high, currently amounting to at least one to two weeks and several thousand euros.
What are you researching specifically at Bosch?
To categorize the mutation status of a tumor, samples pass through numerous process steps that build on each other. Depending on the analysis, this could be more than ten process steps, which — with the intermittent quality checkpoints — take at least two or three days, but generally last a whole week. Although individual steps can be automated — using pipetting robots, for example — this only becomes cost-effective when there is high sample throughput. More and more central laboratories are being set up and used as a result, and all of them need to have high-tech equipment and well-trained specialist personnel.
These central laboratories have disadvantages, however, such as high costs for sample logistics and analysis and comparatively long analysis times. The logistics of sample shipping also causes additional outlay, as samples have to be preserved, which impacts their quality and the information they hold.
Through our research, we are aiming to complement or even replace centralized laboratory operations with devices which integrate those complex process steps and minimize the need for further infrastructure and well-trained personnel at the point of care. This can cut costs, make data available immediately and ensure that all the information contained in the sample can be utilized. Larger sections of the population would be able to benefit from this type of decentralization in genetic testing.
How do you plan to do that?
The main goal of our research is the synergistic combination of MEMS technology with innovative biological process management in a controlled microfluidic environment. We automate and miniaturize diagnostic processes that normally take place in complex medical laboratories and involve many manual steps and many different pieces of equipment. In short, we put a large, well-equipped laboratory onto a small chip (lab-on-chip, or LoC). Our goal is to develop an automated process chain for the NGS analysis of tumor biomarkers at the point of care that can be integrated into a LoC. In this way, we are laying the foundations for a decentralized and more personalized system of medical diagnostics — precisely where it is most urgently needed.
What challenges are you facing in this?
A LoC platform must function under specific conditions like the processibility of microvolumes or the pre-storability of reagents at room temperature amongst many others. However, these limitations open up opportunities to us that others don’t even see. It seems to be discussing the limitations of space for pre-stored reagents in a Lab-on-a-Chip (LoC) cartridge. This is why we are looking into how process steps can be combined and occur in a reduced number of individual reactions. In addition, sequencing errors are key confounding factors for detecting low-frequency genetic variants that are important for molecular cancer diagnosis and treatment monitoring. This can only be complemented by redundant sequence analysis, where sequencing errors in one read can be revealed by the other, based on the principle of “the more the better.” When it comes to an LoC cartridge, which can’t cost too much, this forces us to explore completely new avenues with regard to the process chain. For a product to be commercially viable, it first needs to have low production costs and be suitable for series production.
Looking forward, what is your own personal vision for high-precision, customized tumor treatment?
Even before the 21st century is over, there will be considerably more genetically informed treatments for most types of tumors. These will be treatments that can be read quickly and easily in our genomes, that everyone can afford, and that, above all, significantly improve patients’ quality of life. We can therefore take a lot of the fear of residual disease and tumor recurrence from the patients during the treatment and monitoring phase. All this is accomplished using minimally invasive and considerably more sensitive analyses, known as liquid biopsies.
What do you like best about the research environment at Bosch?
The interdisciplinary working, above all else! After just a few days at Bosch, I was overwhelmed by the huge number of specialist areas with leading experts, all working in a central location. This concentrated knowledge is vital to driving forward successful innovations, as is the close collaboration with visionary strategists and highly professional project directors who work hand in hand with our divisions to get to know market requirements better, for example. The research focus of our team is “Healthcare Solutions”. Experts from the fields of biology, chemistry, physics, microsystem technology, simulation, electronics, mechanical engineering, data, and material science all contribute and share their expertise. I cannot imagine a more stimulating environment with so much passion bundled in one place.
Personalized medicine in practice
The correct medication for each individual – this is not just true for oncology. DNA is examined for other illnesses, too, so patients can receive personalized treatment. The practice of DNA testing was introduced at the Robert Bosch Hospital in Stuttgart (RBK) in cooperation with the Dr. Margarete Fischer-Bosch Institute for Clinical Pharmacology (IKP). Patients’ genomes are examined for gene variants that impact the effects of frequently used medications. The patients then receive this information in a medication pass app that is valid their whole live and is unique to the RBK in Germany. In a well-respected study at the beginning of 2023, researchers from RBK and IKP, in cooperation with an international team, were able to show that the medication pass reduced side effects among patients by around 30 percent.
What are your thoughts on this topic?
Please feel free to share them or to contact me directly.

Author: Dr. Christian Grumaz
Before joining Bosch Research in 2019, Christian Grumaz led the team of a next-generation sequencing unit that provided support for joint research projects with university hospitals, focusing on biomarker discovery and the development of diagnostic assays. Now, at Bosch Research, in his role as an expert in molecular diagnostics and sequencing in the Life Science team, he can contribute to the development of new technologies for next-generation medical diagnostics, especially for point-of-care applications.